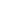China approves 7th self-developed COVID-19 vaccine, can cross-neutralize variants

Medical workers administer COVID-19 vaccine doses to residents at a vaccination site in Tangshan, north China's Hebei Province, May 28, 2021. (Xinhua/Yang Shiyao)
China has approved a seventh domestically developed COVID-19 vaccine to meet increasing demand amid the country's accelerating mass vaccination drive, media reports said on Wednesday, the same day that the country announced it had administered more than 800 million shots.
The latest approved vaccine was developed by the Institute of Medical Biology of the Chinese Academy of Medical Sciences based in Kunming, capital of South Southwest China's Yunnan Province, Science and Technology Daily reported Wednesday.
Phase I/II clinical trials proved the safety and immunogenicity of the vaccine, which can quickly trigger an immune response in recipients, read the report.
At day 14 after two shots, the seroconversion rates of the neutralizing antibodies and anti-S antibodies reached 96 percent and 99.33 percent, respectively. Research also showed that antibodies triggered by the vaccine can cross-neutralize variants of the novel coronavirus, read the report.
The vaccine requires two shots to be administered at an interval of two to four weeks to build immunity in recipients.
Production capacity is estimated to reach 500 million to 1 billion doses per year, the report said.
It is the fifth inactivated COVID-19 vaccine to obtain official approval in China, which will take China's production capacity of inactivated vaccines to about 6 billion per year, Tao Lina, a Shanghai-based vaccine expert, told the Global Times on Wednesday, noting that this should be enough to meet domestic demand.
China's National Health Commission announced on Wednesday that 808.96 million doses of COVID-19 vaccines had been administered as of Tuesday. The announcement came three days after Zeng Yixin, deputy director of the commission, confirmed with media that China aimed to vaccinate about at least 70 percent of its targeted population by the end of this year.
The four other inactivated vaccines approved in China were developed by Sinopharm Group, Sinovac Biotech and Shenzhen Kangtai Biological Products.
A recombinant adenovirus vector COVID-19 vaccine co-developed by Tianjin-based CanSinoBIO and a team led by Chen Wei, an academician at the Chinese Academy of Engineering and a researcher at the Institute of Military Medicine under the Academy of Military Sciences, has been approved. So has a recombinant subunit protein vaccine developed by Anhui Zhifei Longcom. Both have been authorized for emergency use in China.
An inhaled version of the CanSino vaccine has been submitted for emergency use authorization.
To achieve its vaccination goal, China is not only approving more vaccines to expand supply but enlarging its targeted population to give more people access to the vaccines.
Sinovac Chairman Yin Weidong told the media on Friday that the company's vaccine has been authorized for emergency use for children aged between three and 17.
Photos
Related Stories
- Over 800 million COVID-19 vaccine doses administered in China
- Over 794 mln COVID-19 vaccine doses administered across China
- Over 777 mln COVID-19 vaccine doses administered across China
- China encourages international cooperation on vaccine production
- mRNA vaccines greatly reduce COVID-19 infection risk for fully vaccinated: study
Copyright © 2021 People's Daily Online. All Rights Reserved.